Medical device and In Vitro Diagnostic Medical Devices classification in Indonesia has followed ASEAN MEDICAL DEVICE DIRECTIVE (AMDD), which has been aligned to PERMENKES UU NO.62 years 2017. Medical Device classified into 4 risk classes (A,B,C,D based on level of risk) and In Vitro Diagnostic Medical Devices are classified into 4 risk classes (A,B,C,D) influenced by individual risk factors and risks to public health (public health).
In the previous process, after application submission, MoH will take 7 days to do class verification in order to check the class that has been chosen by the application. But, in the future class determination must be done independently by the applicant according to the type of product to be registered. If the applicant chose the wrong class, then MoH will ask applicant to change the class during the 1st evaluation and pay additional fee, if needed. In the classification applicant need to consider some factors including: product name, product type, purpose of use, and how to use.
Below is a diagram describing the changing of the process in determining the class and issuing non-tax revenues/PNBP:
Current condition
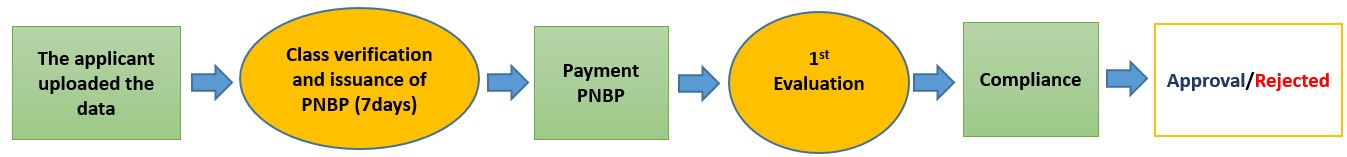
Description: Class determination by registrants, and verified and corrected by the Class Examiner Evaluator
EARLY STAGE
Condition After Change
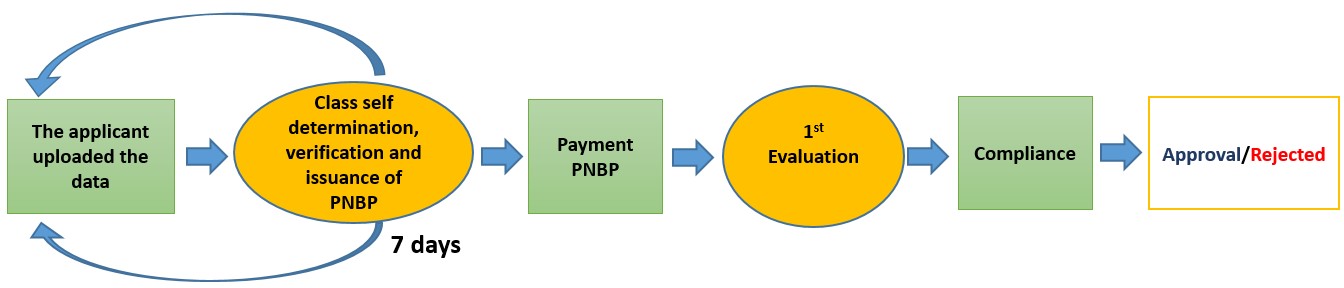
Description: Class self-determination By Applicant. Wrong classification will be returned to the registrants to be revised.
FURTHER STAGES
Condition After Change
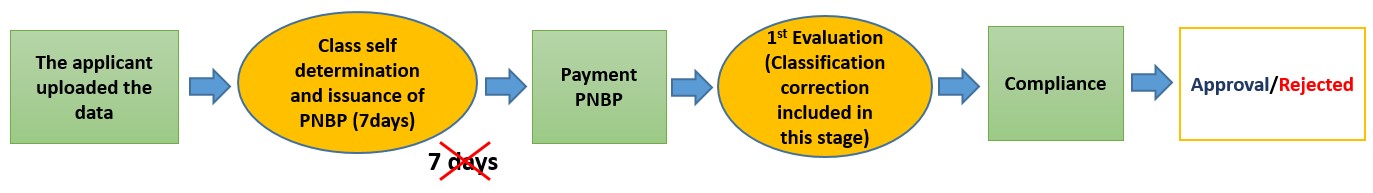
Description: Class self-determination By Applicant. Wrong classification will be regarded as a lack of completeness requirements (TD)
The rule of classification in Indonesia, can be downloaded in MoH website at this link entitled “Pedoman Klasifikasi Alat Kesehatan”.
