Nearly eight months after the implementation of the Medical Device Rules 2017, the Indian Pharmacopoeia Commission (IPC) has brought out the first reference document for medical devices manufactured and sold in the country. Framed mainly on the basis of MD Rules, the 241-page draft rule book is expected to provide manufacturers, licence holders, regulators and healthcare professionals with requisite information on regulatory and technical requirements under one umbrella.
In addition to the new MDR 2017, the document is based on the standards adopted in Indian Pharmacopoeia 2018, British Pharmacopoeia, Japanese Pharmacopoeia, European Pharmacopoeia and the Bureau of Indian Standards.
Currently, India’s medical device sector is dominated by multinational companies, which is evident from the fact that about 80 per cent of the sales are generated by imported devices. Though many multinationals have set up operations in India over the years, a majority of them focus on distribution of imported devices and support functions. The MDR 2017 were framed around the guidelines of the Global Harmonisation Task Force to ensure that the Indian norms are on par with those in vogue globally. Medical devices, both indigenously produced and imported, now have to conform to the best international practices of manufacturing. Against this backdrop, the new reference document will come in handy since the industry is growing at a rapid pace and there is need for an easy-to-access guidebook on regulations and export-import guidelines.
The draft document is likely to be modified as the IPC has requested industry experts and other stakeholders to submit their comments and suggestions on it. Comments can be submitted till September 1, 2018. The document, reviewed by Qualtech, has elaborate sections on classification, registration process, grouping and labelling. More than 20 pages are dedicated for classification of devices and quality parameters, which will be discussed in detail in this and subsequent newsletter editions, once the official version is out.
For starters, the medical device classification in India has been established, as follows:
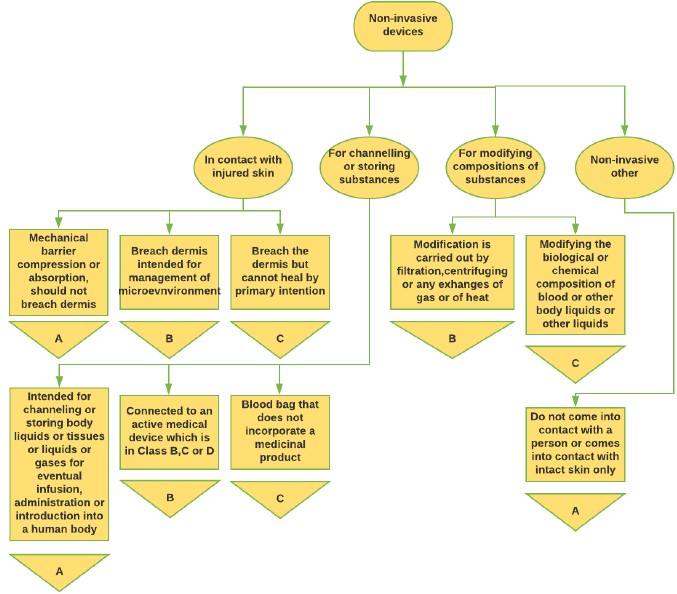
Figure 1: Non-invasive Medical Device classification
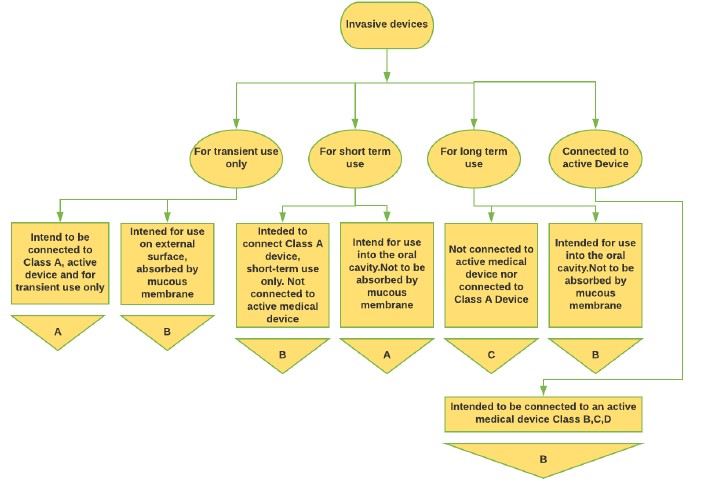
Figure 2: Invasive Medical Device classification
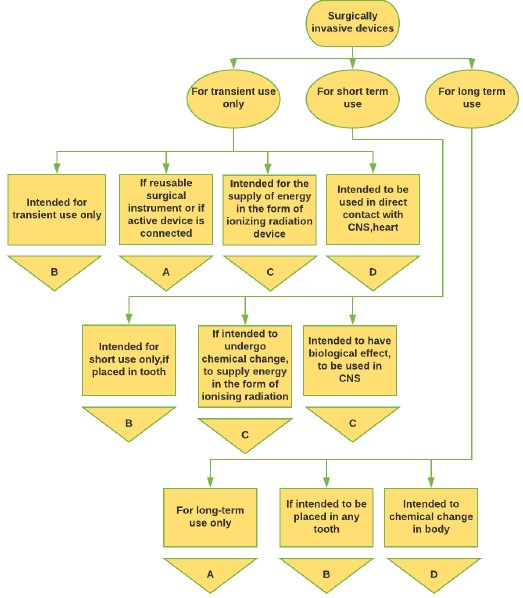
Figure 3: Surgical Medical Device classification
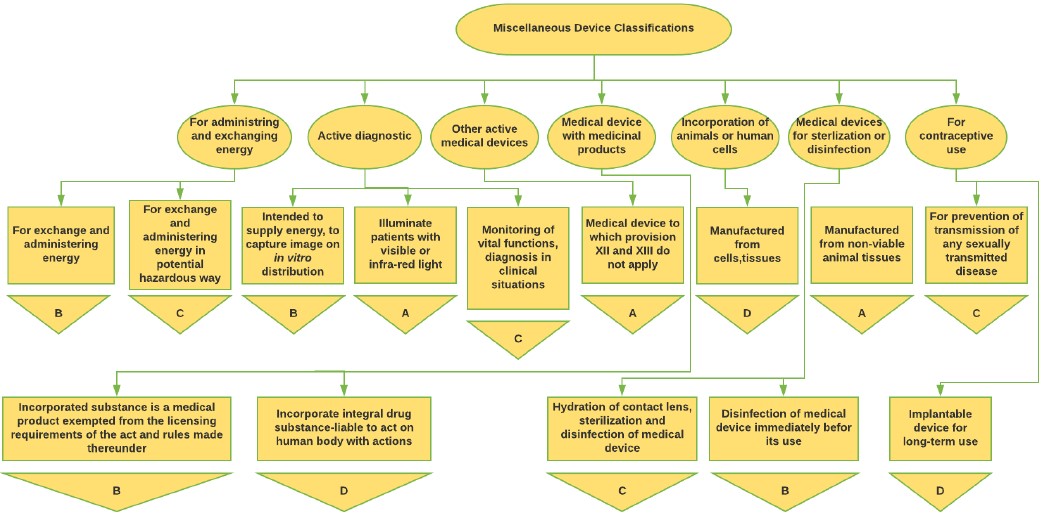
Figure 4: Miscellaneous Medical Device classification
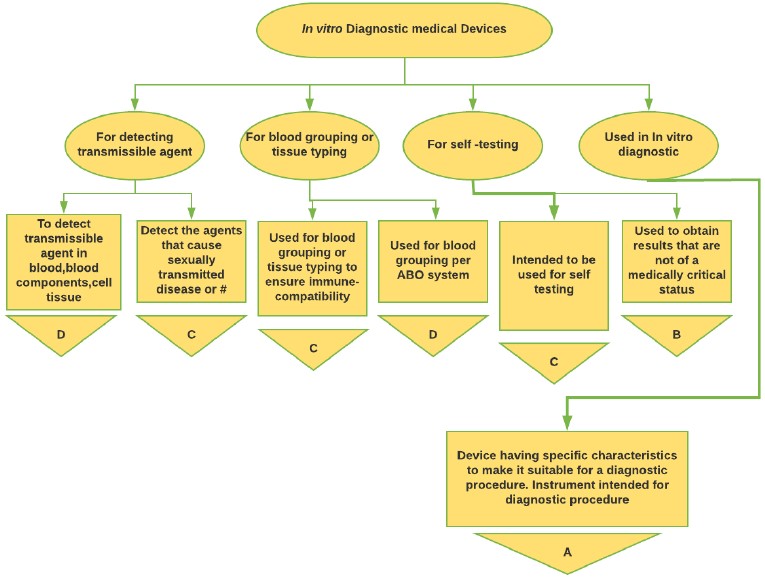
Figure 5: In-vitro Medical Device classification
After categorising medical devices into an appropriate class, next up they have to be placed in an appropriate group, as indicated for medical devices to be manufactured and sold in India, as follows:
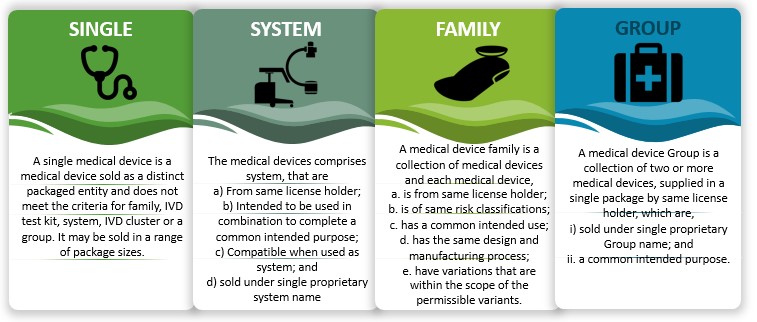
Figure 6: Grouping of medical devices in India
Once this has been established, submission dossier preparation follows. The State Drugs Controller serves as the State Licensing Authority (SLA) and is the competent authority for enforcement of the rules relating to the manufacture of Class A or Class B medical devices and the sale, stocking and exhibition of medical devices and other related functions in India.
On the other hand, Class C and Class D high-risk medical devices are regulated by the Central Licensing Authority (CLA), which oversees the clinical investigation and clinical performance evaluation of medical devices and has other related functions. If a manufacturer intends to manufacture a predicate medical device, the manufacturer must receive approval from the CLA before applying to the SLA. When the device is ready to be registered with CDSCO and imported into India (only applicable to devices in the List of Notified Medical Devices), the following procedure is to be followed:
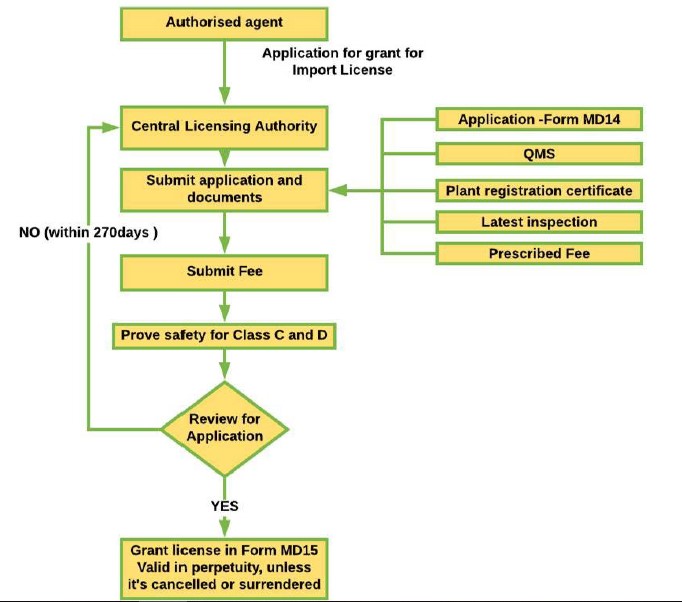
Figure 7: Procedure to apply for import of medical devices
More information on the regulatory process and its related procedures can be further consulted in the draft copy of Guidance Document of Medical Devices by IPC, India. As further developments churn out of the freshly minted medical device regulatory field in India, Qualtech will continue to keep everyone updated to stay abreast of the current changes.
Reference:
Draft copy of Guidance Document of Medical Devices by IPC, India
